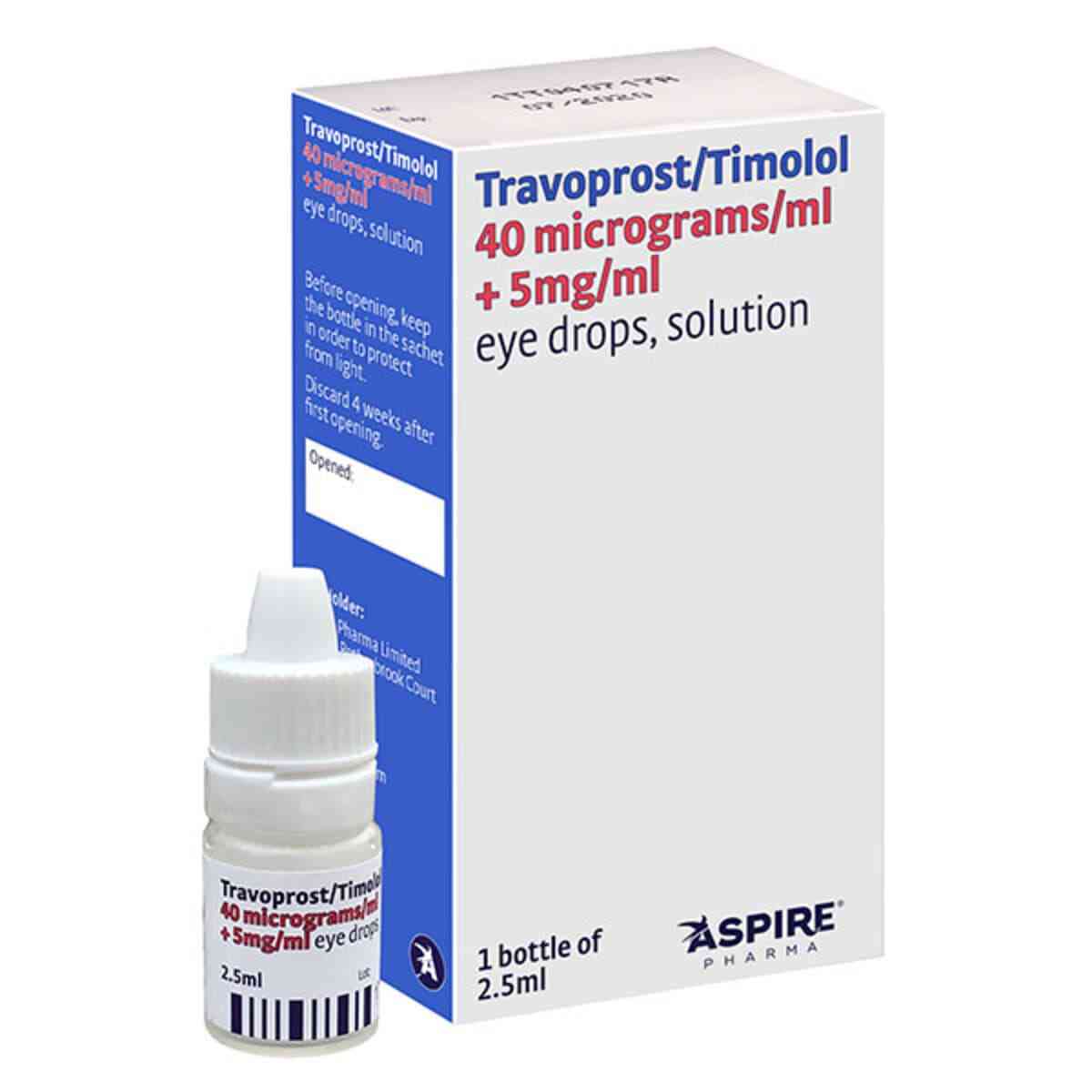
Travoprost Timolol Eye Drops – Duotrav Eye Drops Generic, 2.5ml
Duotrav Eye Drops Generic
Active Ingredient: Travoprost and Timolol
Treatment for Glaucoma
Prescription Product
Vet Prescription Accepted
- Active Ingredient: Travoprost and Timolol
- Treatment for Glaucoma
- Prescription Product
Original price was: £17.50.£11.90Current price is: £11.90.
CompareCompare- Active Ingredient: Travoprost and Timolol
- Treatment for Glaucoma
- Prescription Product
Travoprost Timolol Eye Drops – Duotrav Eye Drops Generic, 2.5ml
What Travoprost Timolol Eye Drops ( Duotrav Eye Drops Generic ) is and what it is used for
Travoprost Timolol eye drops, solution is a combination of two active substances travoprost and timolol. Travoprost is a prostaglandin analogue which works by increasing the outflow of aqueous fluid from the eye, which lowers its pressure. Timolol is a beta-blocker which works by reducing the production of fluid within the eye.
Travoprost and timolol work together to reduce pressure within the eye.
Travoprost Timolol eye drops, Duotrav Eye Drops Generic are used to treat high pressure in the eye in adults, including the elderly. This pressure can lead to an illness called glaucoma.
What is Glaucoma?
Glaucoma is a common eye condition where the optic nerve, which connects the eye to the brain, becomes damaged.
It’s usually caused by fluid building up in the front part of the eye, which increases pressure inside the eye.
Your eye contains a clear, watery liquid that feeds the inside of the eye. Liquid is constantly being drained out of the eye and new liquid is made to replace this. If the liquid cannot drain out quickly enough, the pressure inside the eye builds up. This medicine works by increasing the amount of liquid that is drained. This reduces the pressure inside the eye. If the high pressure is not reduced, it could lead to a disease called glaucoma and eventually damage your sight.
Glaucoma can lead to loss of vision if it’s not diagnosed and treated early.
It can affect people of all ages, but is most common in adults in their 70s and 80s.
Glaucoma does not usually cause any symptoms to begin with.
It tends to develop slowly over many years and affects the edges of your vision (peripheral vision) first.
For this reason, many people do not realise they have glaucoma, and it’s often only picked up during a routine eye test.
If you do notice any symptoms, they might include blurred vision, or seeing rainbow-coloured circles around bright lights.
Both eyes are usually affected, although it may be worse in 1 eye.
Very occasionally, glaucoma can develop suddenly and cause:
Visit an opticians or a GP if you have any concerns about your vision.
If you have glaucoma, early diagnosis and treatment can help stop your vision getting worse.
Without treatment, glaucoma can eventually lead to blindness.
If you develop symptoms of glaucoma suddenly, go to your nearest eye casualty unit or A&E as soon as possible.
This is a medical emergency that may require immediate treatment.
There are several different types of glaucoma.
The most common is called primary open angle glaucoma. This tends to develop slowly over many years.
It’s caused by the drainage channels in the eye becoming gradually clogged over time.
Other types of glaucoma include:
- acute angle closure glaucoma – an uncommon type caused by the drainage in the eye becoming suddenly blocked, which can raise the pressure inside the eye very quickly
- secondary glaucoma – caused by an underlying eye condition, such as inflammation of the eye (uveitis)
- childhood glaucoma (congenital glaucoma) – a rare type that occurs in very young children, caused by an abnormality of the eye
Glaucoma can occur for a number of reasons.
Most cases are caused by a build-up of pressure in the eye when fluid is unable to drain properly.
This increase in pressure then damages the nerve that connects the eye to the brain (optic nerve).
It’s often unclear why this happens, although certain things can increase the risk, including:
- your age – glaucoma becomes more common as you get older
- your ethnicity – people of African, Caribbean or Asian origin are at a higher risk
- your family history – you’re more likely to develop glaucoma if you have a parent or sibling with the condition
- other medical conditions – such as short-sightedness, long-sightedness and diabetes
It’s not clear whether you can do anything to prevent glaucoma, but having regular eye tests should pick it up as early as possible.
The treatment recommended for you will depend on the type of glaucoma you have, but the options are:
- eyedrops – to reduce the pressure in your eyes
- laser treatment – to open up the blocked drainage tubes or reduce the production of fluid in your eyes
- surgery – to improve the drainage of fluid
You’ll also probably need regular appointments to monitor your condition and check the treatment is working.
Further Information on Glaucoma
Duotrav Eye Drops Generic Reviews
After using Duotrav Eye Drops Generic, it’s helpful to let others know about your experience. Reviews of an item help other users know that medicines received have helped the condition it is claimed for, how well the treatment worked or any issues to be aware of. We invite our users to leave a review of both their treatment and of the service provided. Click on the reviews tab to see if there has been feedback on this item.
Price of Duotrav Eye Drops Generic in UK
Where to buy Duotrav Eye Drops Generic
Duotrav Eye Drops Generic is available to buy with a prescription at Dock Pharmacy Essex UK, UK Online Pharmacy.
You can buy Travoprost Timolol Eye Drops uk with a private prescription or with a vets prescription.
Azarga eye drops uk patient Information leaflet
Further Information Glaucoma
Brand
DUOTRAV
How to use
How to use Travoprost Timolol Eye Drops
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is one drop in the affected eye or eyes once a day in the morning or in the evening. Use at the same time each day.
Only use Travoprost/Timolol in both eyes if your doctor told you to do so.
Only use Travoprost/Timolol as eye drops.
Instructions for use
- Immediately before using a bottle for the first time, open the sachet (picture 1). Take the bottle out and write the date of opening on the label in the space provided.
- Make sure you have a mirror available.
- Wash your hands.
- Twist off the bottle cap.
- Hold the bottle, pointing down, between your thumb and fingers.
- Tilt your head back. Pull your lower eyelid down with a clean finger, until there is a ‘pocket’ between the eyelid and your eye. The drop will go in here (picture 2).
- Bring the bottle dropper close to the eye.
Use the mirror if it helps.
- Do not touch your eye or eyelid, the surrounding areas or other surfaces with the dropper.
It could infect the drops. - Gently squeeze the bottle to release one drop of this medicine at a time (picture 3). If a drop misses your eye, try again.
- After using this medicine, press your finger into the corner of your eye, by your nose for 2 minutes (picture 4). This helps to stop this medicine getting into the rest of the body.
- If you have to use Travoprost/Timolol in both eyes, repeat the above steps for your other eye.
- Close the bottle cap firmly immediately after use.
- Only use one bottle of this medicine at a time. Do not open the sachet until you need to use the bottle.
- You must throw away the bottle 4 weeks after you first opened it, to prevent the risk of infections, and start a new bottle.
Use Travoprost/Timolol for as long as your doctor has told you to.
If you use more Travoprost/Timolol than you should
If you use more Travoprost/Timolol than you should, rinse it all out with warm water. Do not put in any more drops until it is time for your next regular dose.
If you forget to use Travoprost/Timolol
If you forget to use Travoprost/Timolol, continue with the next dose as planned. Do not take a double dose to make up for a forgotten dose. The dose should not exceed one drop daily in the affected eye(s).
If you stop using Travoprost/Timolol
If you stop using Travoprost/Timolol without speaking to your doctor, the pressure in your eye will not be controlled, which could lead to loss of sight.
If you are using other eye drops in addition to Travoprost/Timolol, leave at least 5 minutes between applying Travoprost/Timolol and the other drops.
If you wear soft contact lenses, do not use the drops with your lenses in. After using the drops wait 15 minutes before putting your lenses back in.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Product Details
What you need to know before you use Travoprost Timolol Eye Drops – Duotrav Eye Drops Generic
Do not use Travoprost Timolol eye drops
- if you are allergic to travoprost, timolol, or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to any prostaglandins or beta-blockers.
- if you have now or have had in the past respiratory problems such as asthma, severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long standing cough), or other types of breathing problems.
- if you have severe hay fever.
- if you have a slow heartbeat, heart failure or a disorder of heart rhythm (irregular heartbeat).
- if the surface of your eye is cloudy.
Ask your doctor for advice if any of these applies to you.
Warnings and precautions
Talk to your doctor before using Travoprost/Timolol, if you have now or have had in the past
- coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure, low blood pressure.
- disturbances of heart rate such as slow heartbeat.
- breathing problems, asthma or chronic obstructive pulmonary disease.
- poor blood circulation disease (such as Raynaud’s disease or Raynaud’s syndrome).
- diabetes (as timolol may mask signs and symptoms of low blood sugar).
- overactivity of the thyroid gland (as timolol may mask signs and symptoms of thyroid disease).
- myasthenia gravis (chronic neuromuscular weakness).
- cataract surgery.
- eye inflammation.
If you need to have any type of surgery, tell your doctor that you are using Travoprost/Timolol, as timolol may change the effects of some medicines used during anaesthesia.
If you get any severe allergic reaction (skin rash, redness and itching of the eye) while using Travoprost/Timolol, whatever the cause, adrenaline treatment may not be as effective. It is therefore important to tell the doctor that you are using Travoprost/Timolol, when you are to receive any other treatment.
Travoprost/Timolol may change the colour of your iris (the coloured part of your eye). This change may be permanent.
Travoprost/Timolol may increase the length, thickness, colour and/or number of your eyelashes and may cause unusual hair growth on your eyelids.
Travoprost may be absorbed through the skin and therefore should not be used by women who are pregnant or are attempting to become pregnant. If any of the medicine comes into contact with the skin then it should be washed off straight away.
Children and adolescents
Travoprost/Timolol is not to be used by children and adolescents under 18 years of age.
Other medicines and Travoprost/Timolol
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Travoprost/Timolol can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use
- medicines to lower blood pressure,
- heart medicines including quinidine (used to treat heart conditions and some types of malaria),
- medicines to treat diabetes or the antidepressants fluoxetine or paroxetine.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Do not use Travoprost/Timolol, if you are pregnant, unless your doctor considers it necessary. If you could get pregnant, you must use adequate contraception, whilst you use the medicine.
Do not use Travoprost/Timolol if you are breast-feeding. This medicine may get into your milk.
Driving and using machines
You may find that your vision is blurred for a time just after you use Travoprost/Timolol. Do not drive or use machines until this has worn off.
Travoprost/Timolol contains benzalkonium chloride and macrogolglycerol hydroxystearate 40.
This medicine contains 150 micrograms benzalkonium chloride in each ml of solution.
Benzalkonium chloride may be absorbed by soft contact lenses and may change the colour of the contact lenses. You should remove contact lenses before using this medicine and put them back in 15 minutes afterwards.
Benzalkonium chloride may also cause eye irritation, especially if you have dry eyes or disorders of the cornea (the clear layer at the front of the eye). If you feel abnormal eye sensation, stinging or pain in the eye after using this medicine, talk to your doctor.
This medicine contains macrogolglycerol hydroxystearate 40, which may cause skin reactions.
How to store Travoprost Timolol eye drops
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, sachet and bottle after EXP. The expiry date refers to the last day of that month.
Before opening, this medicine does not require any special temperature storage conditions. Keep the bottle in the sachet in order to protect from light.
After first opening, this medicine does not require any special storage conditions.
You must throw away the bottle 4 weeks after you first opened it to prevent the risk of infections. Each time you start a new bottle write down the date you open it in the spaces on the bottle label and carton.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Side Effects
Like all medicines, Travoprost Timolol eye drops can cause side effects, although not everybody gets them.
You can usually carry on using the drops, unless the effects are serious. If you are worried, talk to a doctor or pharmacist. Do not stop using Travoprost/Timolol without speaking to your doctor.
Very common
(may affect more than 1 in 10 people):
Effects in the eye: eye redness
Common (may affect up to 1 in 10 people):
Effects in the eye: eye surface inflammation with surface damage, eye pain, blurred vision, abnormal vision, dry eye, itchy eye, eye discomfort, signs and symptoms of eye irritation (e.g. burning, stinging).
Uncommon
(may affect up to 1 in 100 people):
Effects in the eye: inflammation of the eye surface, inflammation of the eyelid, swollen conjunctiva, increased growth of eyelashes, iris inflammation, eye inflammation, sensitivity to light, reduced vision, tired eyes, eye allergy, eye swelling, increased tear production, eyelid redness, eyelid colour change, skin darkening (around the eye).
General side effects: allergic reaction to active substance, dizziness, headache, increased or decreased blood pressure, shortness of breath, excessive hair growth, drip at the back of the throat, skin inflammation and itching, decreased heart rate.
Rare (may affect up to 1 in 1,000 people):
Effects in the eye: thinning of the eye surface, inflammation of the eyelid glands, broken blood vessel in the eye, eyelid crusting, abnormally positioned eyelashes, abnormal growth of lashes.
General side effects: nervousness, irregular heart rate, loss of hair, voice disorders, difficulty breathing, cough, throat irritation, hives, abnormal liver blood tests, skin discolouration, thirst, tiredness, discomfort inside of nose, coloured urine, pain in hands and feet.
Not known (frequency cannot be estimated from the available data):
Effects in the eye: droopy eyelid (making the eye stay half closed), sunken eyes (eyes appear more inset), changes in the colour of the iris (coloured part of the eye), hallucination.
General side effects: rash, heart failure, chest pain, stroke, fainting, depression, asthma, increased heart rate, numbness or tingling sensation, palpitations, swelling in the lower limbs, bad taste.
Additionally:
Travoprost/Timolol is a combination of two active substances, travoprost and timolol. Like other medicines administered to the eyes, travoprost and timolol (a beta-blocker) are absorbed into the blood. This may cause side effects similar to those seen when beta-blocking medicines are administered by mouth or by injection. The incidence of side effects after administration to the eyes is lower than after administration by mouth or by injection.
The side effects listed below include reactions seen with the class of beta-blockers used for treating eye conditions or reactions seen with travoprost alone:
Effects in the eye: inflammation of the eyelid, inflammation in the cornea, detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, decreased corneal sensitivity, corneal erosion (damage to the front layer of the eyeball), double vision, eye discharge, swelling around the eye, eyelid itching, outward turning of eyelid with redness, irritation and excessive tears, blurred vision (sign of clouding of the eye lens), swelling of a section of the eye (uvea), eczema of the eyelids, halo vision, decreased eye sensation, pigmentation inside the eye, dilated pupils, change in eyelash colour, change in the texture of the eyelashes, abnormal field of vision.
General side effects:
- Ear and labyrinth disorders: dizziness with spinning sensation, ringing in the ears.
- Heart and circulation: slow heart rate, palpitations, oedema (fluid build-up), changes in heartbeat rhythm or speed, congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build-up), a type of heart rhythm disorder, heart attack, low blood pressure, Raynaud’s phenomenon, cold hands and feet, reduced blood supply to the brain.
- Respiratory: constriction of the airways in the lungs (predominantly in patients with pre-existing disease), runny or stuffy nose, sneezing (due to allergy), difficulty breathing, nose bleed, nasal dryness.
- Nervous system and general disorders: difficulty sleeping (insomnia), nightmares, memory loss, loss of strength and energy, anxiety (excessive emotional distress).
- Gastrointestinal: taste disturbances, nausea, indigestion, diarrhoea, dry mouth, abdominal pain, vomiting and constipation.
- Allergy: increased allergic symptoms, generalized allergic reactions including swelling beneath the skin that can occur in areas such as the face and limbs and can obstruct the airway which may cause difficulty swallowing or breathing, localized and generalized rash, itchiness, severe sudden life-threatening allergic reaction.
- Skin: skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis, peeling skin, abnormal hair texture, inflammation of the skin with itchy rash and redness, hair colour change, loss of eyelashes, itching, abnormal hair growth, skin redness.
- Muscular: increases in signs and symptoms of myasthenia gravis (muscle disorder), unusual sensations like pins and needles, muscle weakness/tiredness, muscle pain not caused by exercise, joint pain.
- Renal and urinary disorders: difficulty and pain when passing urine, involuntary leakage of urine.
- Reproduction: sexual dysfunction, decreased libido.
- Metabolism: low blood sugar levels, increase in prostate cancer marker.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store). By reporting side effects you can help provide more information on the safety
Ingredient
What Travoprost Timolol eye drops contains
- The active substances are travoprost and timolol. Each ml of solution contains 40 micrograms of travoprost and 5mg of timolol (as timolol maleate).
- The other ingredients are benzalkonium chloride, macrogolglycerol hydroxystearate 40, trometamol, edetate disodium, boric acid (E284), mannitol (E421), sodium hydroxide (for pH adjustment) and water for injections or purified water.
Questions and answers of the customers
There are no questions yet, be the first to ask something for this product.
Other Products From This Seller
Original price was: £63.00.£15.95Current price is: £15.95.
Dunhill Icon Racing Green Eau de Parfum Sprays, 100ml Embrace the thrill of sophistication with Dunhill Icon Racing Green Eau de Parfum, 100ml, a fresh and woody fragrance for men inspired by the spirit of speed and elegance. This luxurious scent from Dunhill blends invigorating citrus top notes with a warm woody base, making it […]
Learn MoreOriginal price was: £63.00.£15.95Current price is: £15.95.
- Availability: in stock
Original price was: £45.00.£18.95Current price is: £18.95.
Guy Laroche Drakkar Noir Eau de Toilette Spray, 100ml Bold and Aromatic Men’s Fragrance Exude timeless masculinity with Guy Laroche Drakkar Noir Eau de Toilette Spray, 100ml, an iconic men’s fragrance that blends boldness and sophistication. Launched by Guy Laroche, this classic scent remains a favorite for men seeking a powerful and confident aroma. Drakkar […]
Learn MoreOriginal price was: £45.00.£18.95Current price is: £18.95.
- Availability: in stock
Original price was: £49.00.£24.95Current price is: £24.95.
Dunhill Desire Blue for Men Eau de Toilette, 100 ml Dive into elegance and freshness with Dunhill Desire Blue for Men Eau de Toilette, 100ml, a fresh and aquatic fragrance that embodies sophistication and charm. Crafted for the modern man, this invigorating scent blends crisp citrus, aromatic herbs, and warm woody undertones to create a […]
Learn MoreOriginal price was: £49.00.£24.95Current price is: £24.95.
- Availability: in stock
Original price was: £33.95.£14.95Current price is: £14.95.
David Beckham Classic Eau de Toilette 90ml Sophisticated and Woody Fragrance for Men Step into timeless sophistication with David Beckham Classic Eau de Toilette, 90ml, a woody and citrus fragrance for men that exudes confidence and style. Designed for the modern gentleman, this iconic scent captures the elegance and charisma synonymous with David Beckham. Whether […]
Learn MoreOriginal price was: £33.95.£14.95Current price is: £14.95.
- Availability: in stock
Original price was: £40.00.£19.99Current price is: £19.99.
ARMAF Club De Nuit Intense Man Eau De Parfum, 30ml Bold and Long-Lasting Fragrance for Men Elevate your presence with ARMAF Club De Nuit Intense Man Eau De Parfum, 30ml, a bold and woody fragrance for men that exudes sophistication and confidence. Crafted for the modern gentleman, this luxurious scent combines fresh citrus, aromatic spices, […]
Learn MoreOriginal price was: £40.00.£19.99Current price is: £19.99.
- Availability: in stock
Original price was: £35.00.£18.95Current price is: £18.95.
Calvin Klein CK IN2U Him Eau De Toilette 50ml Fresh, Spicy, and Woody Fragrance Celebrate youth, energy, and spontaneity with Calvin Klein CK IN2U Him Eau De Toilette, 50ml, a fresh and spicy fragrance for men. This dynamic scent perfectly balances invigorating citrus top notes with warm woody undertones, embodying the spirit of modern masculinity. […]
Learn MoreOriginal price was: £35.00.£18.95Current price is: £18.95.
- Availability: in stock
Original price was: £66.00.£19.95Current price is: £19.95.
Calvin Klein Man Eau de Toilette 100ml Fresh, Bold, and Woody Fragrance for Men Define your masculinity with Calvin Klein Man Eau de Toilette, 100ml, a fresh and woody fragrance for men that embodies confidence, sophistication, and modernity. This bold scent blends crisp and spicy notes with warm undertones, making it an ideal choice for […]
Learn MoreOriginal price was: £66.00.£19.95Current price is: £19.95.
- Availability: in stock
Original price was: £38.00.£22.99Current price is: £22.99.
BURBERRY Weekend For Men Edt Spray, 30 ml Fresh and Casual Fragrance Relax in style with BURBERRY Weekend For Men Eau de Toilette Spray, 30ml, a fresh and citrusy fragrance for men that perfectly embodies casual elegance. Designed for modern, relaxed men, this scent balances refreshing citrus top notes with warm, woody undertones. Ideal for […]
Learn MoreOriginal price was: £38.00.£22.99Current price is: £22.99.
- Availability: in stock
Original price was: £36.00.£24.95Current price is: £24.95.
BURBERRY Touch For Men Eau de Toilette 30ml Fresh and Woody Fragrance Experience the refined elegance of BURBERRY Touch For Men Eau de Toilette, 30ml, a fresh and woody fragrance for men that captures the essence of modern masculinity. Perfectly balanced with refreshing and warm notes, this iconic scent is ideal for everyday wear and […]
Learn MoreOriginal price was: £36.00.£24.95Current price is: £24.95.
- Availability: in stock
Original price was: £9.95.£4.95Current price is: £4.95.
Aqua Man DC Pour Homme Eau De Parfum 100ml Bold and Fresh Aquatic Fragrance for Men Unleash your inner hero with Aqua Man DC Pour Homme Eau De Parfum, 100ml, a fresh and aquatic fragrance for men that embodies strength, confidence, and charisma. Inspired by the iconic DC superhero Aqua Man, this invigorating scent is […]
Learn MoreOriginal price was: £9.95.£4.95Current price is: £4.95.
- Availability: in stock